Products
Celularity Tissue Products Sales Sheet
A fully integrated, purpose-built manufacturing and research center $80M investment in platform-based cGMP/cGTP manufacturing Optimized, product-specific CMC, QA/QC and manufacturing processes accelerate product development, production and commercialization Rapidly scalable, end-to-end supply chain Greater efficiency and economy than can be achieved through outsourcing to contract manufacturing organizations (CMOs) alone. ...
Celularity Interfyl Surgeon
Interfyl® Connective Tissue Matrix (CTM) Your first choice in healing soft tissue deficits and injuries. Highly adaptable and effective at replacing or supplementing damaged tissue, across a wide-variety of conditions. Celularity Interfyl Surgeon
Celularity Bone Fusion Summary
The Use Of Interfyl, When Mixed With Cancellous Autograft, May Accelerate Healing By Offsetting Some Negative Patient Health Factors. Autogenous bone graft is the gold standard for reconstruction of bone defects and the preferred adjunctive tissue for arthrodesis procedures. But, there are limitations to this method of treatment Celularity Bone ...
Celularity Biovance 3L detail aide - new
Indications For Use Biovance 3L is an allograft intended for use as a biological membrane covering that provides an extracellular matrix. As a barrier membrane, Biovance 3L is intended to protect the underlying tissue and create a barrier between the tissue plane boundaries. Indications include, but are not limited to, ...
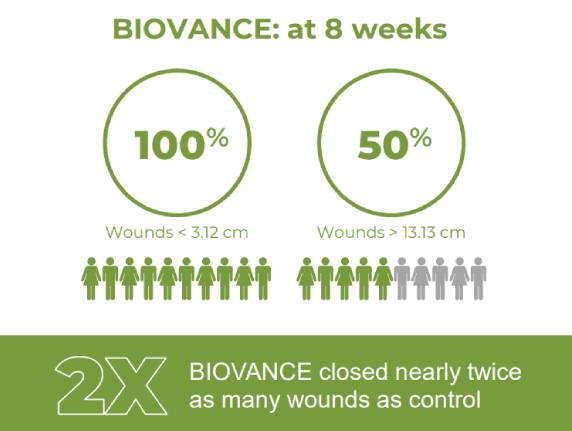
Celularity Real World Wound Study Summary
The aim of this observational study was to gain experience in the use and performance of BIOVANCE® versus standard of care (SOC) in a real-world wound population. A broad range of partial and full thickness wounds were studied across a mix of patient types. Eligibility for inclusion included any ...
Biovance Patient Brochure
Hard-to-Heal Wound A chronic wound is one that does not heal as it should. This may happen because of certain conditions like heart disease and diabetes, which can cause poor circulation. If the wound is not healed, it may lead to other problems, such as decreased quality of life. Biovance ...
Tissue Products Sales Sheet
A fully integrated, purpose-built manufacturing and research center $80M investment in platform-based cGMP/cGTP manufacturing Optimized, product-specific CMC, QA/QC and manufacturing processes accelerate product development, production and commercialization Rapidly scalable, end-to-end supply chain Greater efficiency and economy than can be achieved through outsourcing to contract manufacturing organizations (CMOs) alone. ...
Bone Fusion Summary
The Use Of Interfyl, When Mixed With Cancellous Autograft, May Accelerate Healing By Offsetting Some Negative Patient Health Factors. Autogenous bone graft is the gold standard for reconstruction of bone defects and the preferred adjunctive tissue for arthrodesis procedures. But, there are limitations to this method of treatment Celularity Bone ...
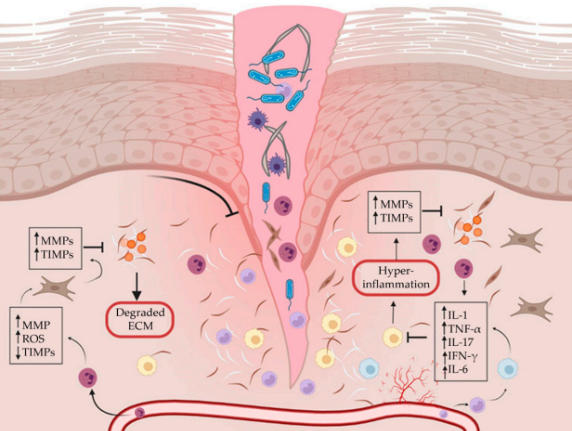
Placental Derived Biomaterials and Their Application to Wound Healing
Abstract: Chronic wounds are associated with considerable patient morbidity and present a significant economic burden to the healthcare system. Often, chronic wounds are in a state of persistent inflammation and unable to progress to the next phase of wound healing. Placental-derived biomaterials are recognized for their biocompatibility, biodegradability, angiogenic, anti-inflammatory, ...
18-CLI-2210 CS Interfyl Pdf Dwnlds B
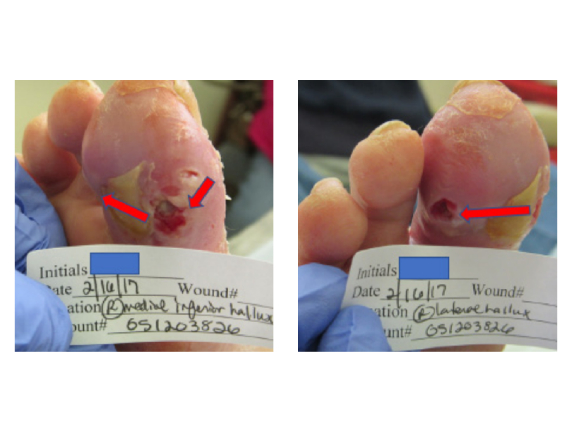
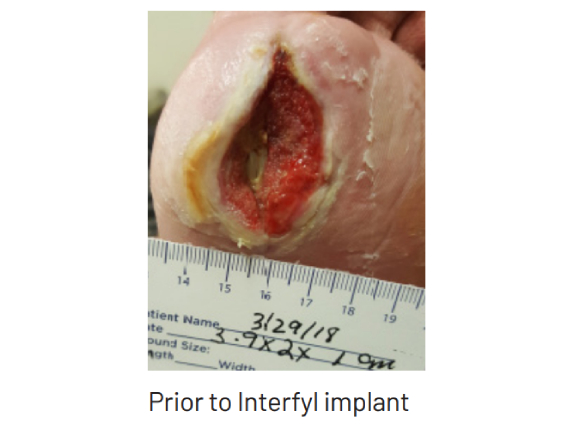
Presentation Background: Diabetic neuropathic plantar hallux ulcer of the right foot Initial treatment: Treated initially with oral Levofloxacin 30 days until cultures negative for bacteria. Local care with silver hydrogel and hydrofiber packing Findings: Tunneling ulcer through medial to plantar to lateral hallux. Tendon exposed. Bone palpable but not visible. Loss ...
18-CLI-2210 CS Interfyl Pdf Dwnlds C
Presentation Background: Diabetic neuropathic plantar foot ulcer sustained over 1 year Initial treatment: Conservative: hydrogel, collagen, foam bandage. No offloading Surgical: partial sesamoidectomy with wide excisional wound debridement and closure Findings: Vascular studies showing adequate perfusion. Cultures negative Pre-op x-rays: Showed hypertrophic sesamoid. Negative for osteomyelitis 18-CLI-2210 CS Interfyl Pdf ...
TrackingLetter Interfyl
FDA Tracking Requirements for Human Tissue-based Products Thank you for choosing INTERFYL®, an allogeneic decellularized particulate human placental connective tissue matrix, which is regulated by the United States Food and Drug Administration (FDA) as a human tissue-based product. TrackingLetter Interfyl