Products
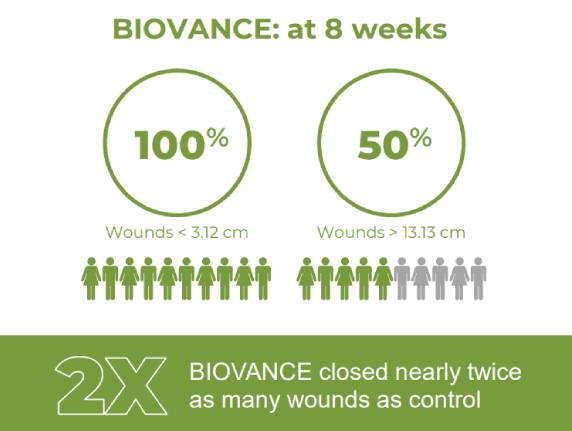
Celularity Real World Wound Study Summary
The aim of this observational study was to gain experience in the use and performance of BIOVANCE® versus standard of care (SOC) in a real-world wound population. A broad range of partial and full thickness wounds were studied across a mix of patient types.
- Eligibility for inclusion included any patient that would benefit from treatment
- Unlike other chronic wound prospective, randomized, controlled trials, there were no limits on patients’ age, baseline wound size or co-existing conditions
- Key comorbidities included: arterial insufficiency, autoimmune disease, diabetes, and edema/lymphedema
‹ Back